Polyester biodegradability: importance and potential for optimisation
The existing polyolefin plastics are not adequately suited for closed-loop recycling; however, biodegradable plastics may prove an effective solution to the accumulation of plastic. Even in the presence of effective waste collection systems, plastics frequently find their way into the environment through littering, leading to significant environmental accumulation if they are not biodegradable. It is estimated that 6–17 million tons of plastic waste accumulates in the environment annually. The improper disposal of plastic waste has the potential to result in environmental contamination and pose a threat to the viability of ecosystems. Biodegradable polyesters, in theory, possess the capacity for recycling. Despite the inherent challenges associated with mechanical recycling, these materials can undergo chemical breakdown into monomers for repolymerization.1
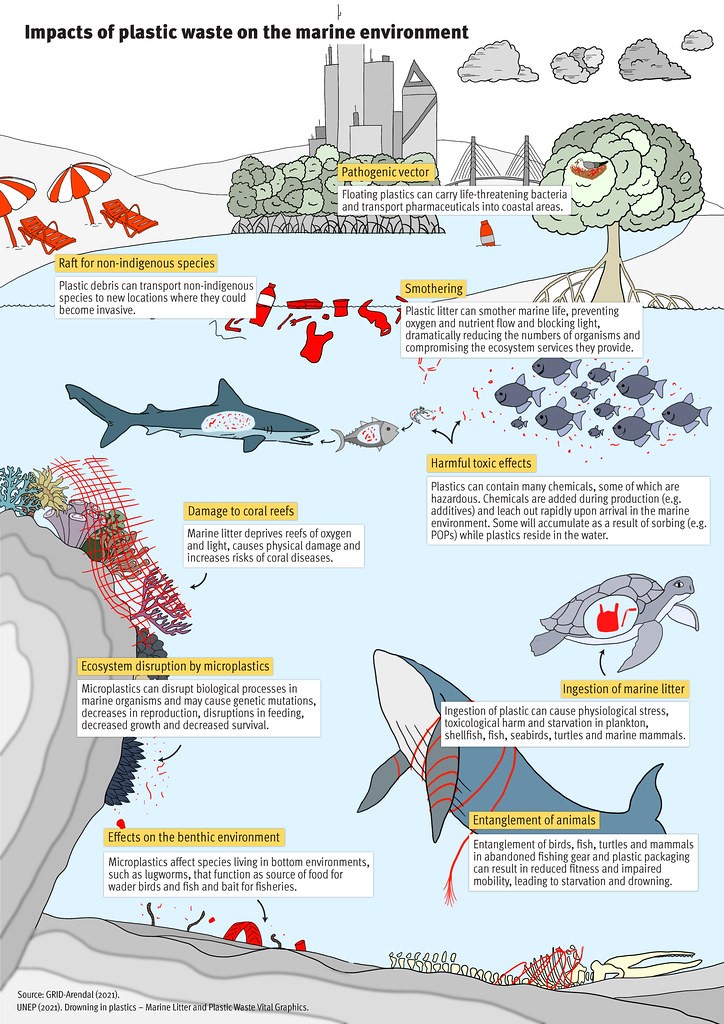
Definition of biodegradation
The International Union of Pure and Applied Chemistry (IUPAC) defines a biodegradable polymer as “polymer susceptible to degradation by biological activity, with the degradation accompanied by a lowering of its molar mass”.2
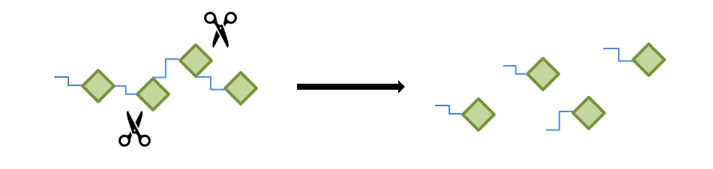
Depolymerization: polyesters hydrolysis
Depolymerization, like hydrolysis of polyesters, is the cleavage of bonds within the polymer chains, reducing the molecular weight of the polymer and releasing small molecules. The energy required for this cleavage can come from a variety of sources, including thermal, light, mechanical, chemical and/or biological energy. Polyesters are the largest group of biodegradable thermoplastics produced on industrial scale. Polyesters can be recycled by two distinct processes: mechanical and chemical. In comparison to polyolefins, the esterification process is chemically reversible, allowing for the reversal of the reaction via hydrolysis or alcoholysis. This enables the breakdown of polyesters into monomers, which can then be repolymerised (optionally after purification). This recycling process is therefore a closed loop.
References
(1) Wang, Y.; Putten, R.-J. van; Tietema, A.; Parsons, J. R.; Gruter, G.-J. M. Polyester Biodegradability: Importance and Potential for Optimisation. Green Chem. 2024, 26 (7), 3698–3716. https://doi.org/10.1039/D3GC04489K.
(2) Horie, K.; Barón, M.; Fox, R. B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; Stepto, R. F. T.; Tabak, D.; Vohlídal, J.; Wilks, E. S.; Work, W. J. Definitions of Terms Relating to Reactions of Polymers and to Functional Polymeric Materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76 (4), 889–906. https://doi.org/10.1351/pac200476040889.
(3) Rafiqah, S. A.; Khalina, A.; Harmaen, A. S.; Tawakkal, I. A.; Zaman, K.; Asim, M.; Nurrazi, M. N.; Lee, C. H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13 (9), 1436. https://doi.org/10.3390/polym13091436.
Links
https://pubs.rsc.org/en/content/articlelanding/2024/gc/d3gc04489k
https://www.grida.no/resources/14899
https://goldbook.iupac.org/terms/view/BT07169
https://www.mdpi.com/2073-4360/13/9/1436
Keywords
Polyester, Depolymerization, Poly(butylene succinate), , Biodegradation, Polyolefins, Recycling